The chemical formula calculator - Find the formula for. Lett 2005 7 4107-4110.

Palladium Ii Bromide Powder Supplier Cas 13444 94 5

Binding Parameters For Palladium Ii Complexes 1 5 On The Fluorescence Download Table

Palladium Ii Bromide Wikipedia
V Steric hindrance at the bonding sites may inhibit or prevent reaction.

Palladium ii bromide. MercuryII bromide HgBr 2 62 1020 MercuryII iodide HgI 2 29 1029 Neodymium carbonate Nd 2 CO 3 3 108 1033 NickelII carbonate NiCO 3 142 107 NickelII hydroxide NiOH 2 548 1016 NickelII iodate NiIO 3 2 471 105 NickelII phosphate Ni 3 PO 4 2 474 1032 PalladiumII. Mgs Cl 2 g MgCl 2 s Mgs Br 2 g MgBr 2 s Reaction of magnesium with acids. Aluminium bromide AlBr 3.
Iii Electron withdrawing groups on the dienophile facilitate reaction. This method tolerates various functional groups. Aluminium arsenate AlAsO 48H 2 O.
The numbers under the column headed CASRN are the Chemical Abstracts Service Registry Numbers for each hazardous substanceThe Statutory Code column indicates the statutory source for designating each substance as a CERCLA hazardous substance. PalladiumII is the only other cation which forms a precipitate with this reagent. ActiniumIII oxide - Ac 2 O 3.
Yet the factors that dictate nuclearity. Ii The diene component must be able to assume a s-cis conformation. T-Bu 3 2 to an aryl bromide generates a Pd I.
Aluminium carbide Al 4 C 3. ActiniumIII chloride - AcCl 3. Rutherfordium chloride is believed to be RfCl4.
Aluminium arsenide AlAs. Oxidative addition of aryl bromide after dissociation of phosphine from a two-coordinate palladium0 complex bistri-o-tolylphosphinepalladium0 J Am Chem Soc. ActiniumII fluoride - AcF 3.
Aluminium iodide AlI 3. Similarly rutherfordium bromide is more volatile than hafnium bromide. Meta-alkylation with benzyl bromide.
Ionic compounds with the net ionic equations. Aluminium nitride AlN. Aluminium antimonide AlSb.
Aluminium oxide Al 2 O 3. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Optical constants of Cu Copper Johnson and Christy 1972.
Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. A case in point is the stabilization of palladiumI. The use of a newly developed pyridine-based ligand is crucial for relaying the palladium catalyst to the meta position by.
The calculator is found on the right hand panel of the main page. Aluminium boride AlB 2. The calculator can be used to calculate the chemical formula of a range of.
1 indicates that the statutory source is section 311b2 of the Clean Water Act 2 indicates that the source is section 307a. CobaltII preferentially forms a dark brown solution with dimethylglyoxime and excess reagent must be used in its presence. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
Google Scholar b Hartwig JF Paul F. Subsequent Curtius rearrangement in the presence of tetrabutylammonium bromide and zincII triflate and trapping of the isocyanate derivative gives the desired product. PalladiumII chloride also known as palladium dichloride and palladous chloride are the chemical compounds with the formula PdCl 2PdCl 2 is a common starting material in palladium chemistry palladium-based catalysts are of particular value in organic synthesisIt is prepared by the reaction of chlorine with palladium metal at high temperatures.
It condenses around 220C similar to zirconium chloride but more volatile than hafnium chloride and much more volatile than the actinide tetrachlorides. Magnesium is very reactive towards the halogens such as chlorine Cl 2 or bromine Br 2 and burns to form the dihalides magnesiumII chloride MgCl 2 and magnesiumII bromide MgBr 2 respectively. However a few other cations can interfere.
Optical constants of GaAs Gallium arsenide Aspnes et al. The speciation of homogeneous metal catalysts is a key determinant of reactivity efficiency and selectivity. And the symbols of the elements of the Periodic table.
Acidify the solution to be tested with 6 M CH 3 COOH. Iv Electron donating groups on the diene facilitate reaction. PbC 2 O.

Palladium Ii Bromide Premion 99 998 Metals Basis Pd 39 5 Min Thermo Scientific Fisher Scientific

2 Bis Triphenylphosphine Palladium Ii Bromide Benzyl Alcohol 96 849417 41 0
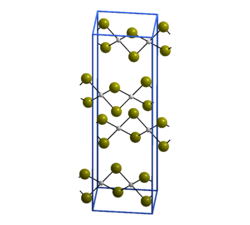
Palladium Ii Bromide Wikipedia

Palladium Ii Chloride Wikiwand

Palladium Ii Iodide Wikipedia
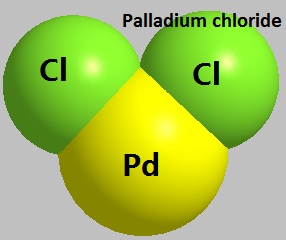
Palladiumchlorid 7647 10 1
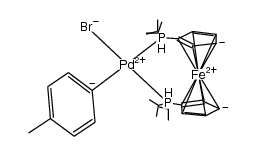
Cas 1154042 98 4 1 1 Bis Diisopropylphosphino Ferrocene P Tolyl Palladium Ii Bromide Chemsrc

Palladium Ii Bromide Wikipedia

